Posted October 4, 2011
UC San Diego neuroscientist Steve Wagner, a previous recipient of two substantial grants from Cure Alzheimer’s Fund (CAF), has been awarded a $1 million NIH “Blueprint” grant for the fast-track development of a promising Alzheimer’s drug therapy.
“This is further validation of our venture model,” says CAF President and CEO Tim Armour. “We’ve always been willing to take considerable risk for the prospect of faster progress. Steve’s project is a sterling example of why our founders adopted this strategy. Thanks in part to CAF’s support for Wagner’s research, the world is now much closer to a promising new class of Alzheimer’s drugs.”
Harvard professor Dr. Rudy Tanzi, Wagner’s collaborator on this project, will serve on the lead development team for the five-year NIH project, which brings together 15 agencies in an unprecedented bid to quickly shepherd drug candidates into clinical trials. The new Blueprint for Neuroscience Research program is the controversial brainchild of NIH Director Francis Collins.
“Blueprint is innovative and critical,” says Tanzi, “and Wagner’s is the perfect inaugural project. I am extremely optimistic about the prospects for this series of drugs, given the very promising preliminary data generated in Steve’s lab and my own at Massachusetts General Hospital.”
Wagner is just as hopeful. “I think this is the final frontier for the beta-amyloid hypothesis,” he says. “We have finally found what we’ve been looking for.”
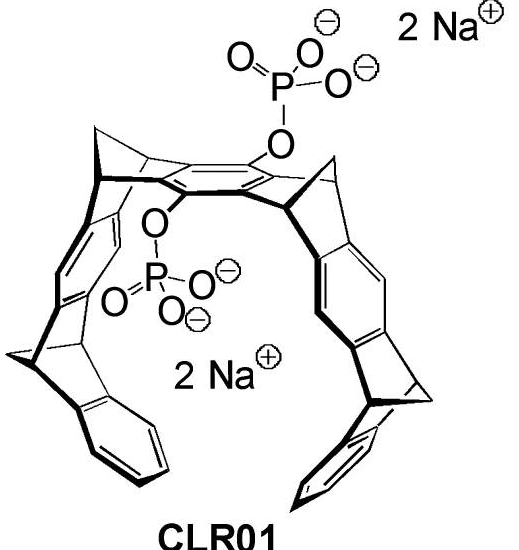
Preventing toxic beta-amyloid protein fragments from accumulating in the brain long has been a goal of Alzheimer’s researchers, because so many studies have shown their direct causal relationship to the disease. Previous compounds aimed at clearing away and preventing the formation of the fragments seemed promising in mice, but caused such major side effects as skin cancer and nausea in humans because they also cleared away vital, non-toxic beta-amyloid. Since those recent failures, Wagner and Tanzi have aimed to develop a compound that would remove only the toxic beta-amyloid. CAF grants in 2009 and 2010 made their research possible.
“I think we may now have that molecule,” Wagner explains. “With the CAF funding, we got through that hurdle. I don’t know how many hurdles are left, but there may not be many. That’s why drug companies are getting real serious about this approach.”
With the large NIH grant now in place, CAF has stepped into a new supporting role, augmenting Wagner’s research with an additional $150,000 to determine his compound’s precise “mechanism of action”—how it actually works. This particular investigation is not covered by the NIH grant but will be crucial, explains Tanzi, for a future clinical trial application to the FDA.
“CAF is so proud to be a part of this project,” says Armour. “As Wagner and Tanzi carry out their vital work, our superb Research Consortium will continue to keep its eyes out for the most promising and aggressive solution-oriented Alzheimer’s research we can find.”