Posted October 3, 2016

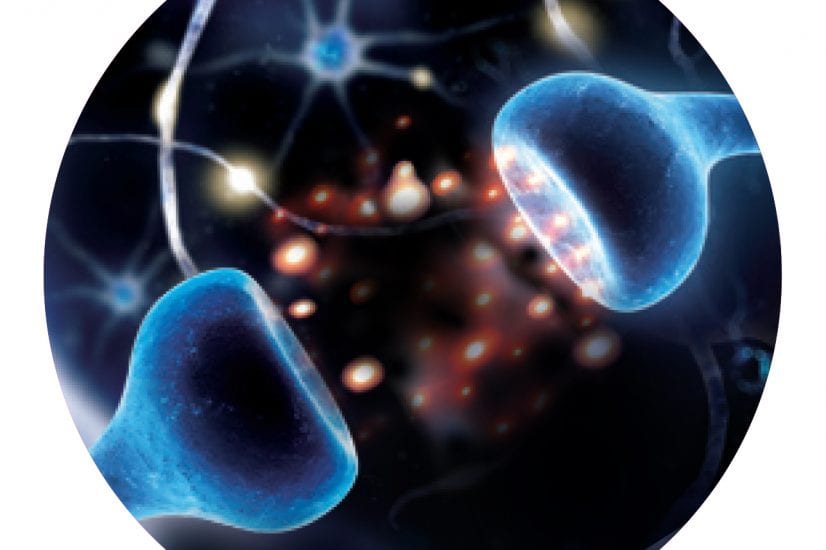
What do the developing brain and the Alzheimer’s brain have in common? Beth Stevens, Ph.D., a developmental neurologist, is investigating an important connection: the loss of synapses, where neurons connect with one another to transmit important signals.
Stevens heads a lab in the F.M. Kirby Neurobiology Center at Children’s Hospital in Boston. She’s a superstar in the field, having received a Presidential Early Career Award for Scientists and Engineers in 2012 and a MacArthur “Genius Award” Foundation Fellowship in 2015. Now, she’s making waves in the Alzheimer’s field. This past May, her lab published a groundbreaking study identifying a pathway that may be responsible for synapse loss in the Alzheimer’s brain, which is closely correlated with cognitive decline.
Synapse loss in development
In the developing brain—from birth to early adolescence—synapse loss occurs normally and frequently. “It’s a ‘use it or lose it’ system,” Stevens explains. “The brain figures out which connections are important and which ones aren’t, based on experience. It prunes the ones it doesn’t need so that it can strengthen the more useful connections.” Synapses allow neurons to communicate with one another, transmitting signals around the brain, enabling us to form thoughts, recall memories, perform motor skills and more. As our brains are molded by synapse growth and loss, our personality and identity also take shape.
Stevens, along with Cure Alzheimer’s Fund Research Consortium member Ben Barres, M.D., Ph.D., published a 2007 paper showing that a protein named “complement” mediates healthy synapse loss. Complement “tags” unnecessary synapses for destruction. In much the same way macrophages devour invading pathogens like bacteria throughout the body, brain cells called microglia devour the complement-marked synapses. This tag-and-destroy activity so important to healthy brain development is also part of the brain’s innate immune system, a first line of defense against infection and disease.
In healthy adults, this complement-pruning pathway largely is turned off. While microglia and complement still play other important roles, they’re no longer trimming synapses on a large scale.
Connections to Alzheimer’s
Stevens first suspected a link between this complement pathway and Alzheimer’s after hearing a lecture by Dennis Selkoe, M.D. Selkoe, a Harvard neurologist previously funded by Cure Alzheimer’s Fund, discussed his finding that synapse loss occurs in Alzheimer’s disease (AD) even before amyloid plaques are detectible. Stevens wondered whether complement might play a role in this very early synapse loss. Collaborating with Soyon Hong, Ph.D., a former graduate student in Selkoe’s lab and now a postdoc in Stevens’ own lab, Stevens launched an investigation to find out whether complement was present in the brains of Alzheimer’s mice.
Their findings supported the hypothesis: complement was upregulated in vulnerable brain regions like the hippocampus in the mice, coating synapses that had been pruned by microglia. Even more intriguing, Stevens and her colleagues found that by disabling or blocking the creation of complement, they could preserve synapses. These protected mice experienced less synapse loss.
Cure Alzheimer’s Fund
The next step was to see whether these findings would translate into human brains. Eager to find funding to continue the experiment, Stevens reached out to fellow researcher Rudy Tanzi, Ph.D., of Harvard Medical School/Massachusetts General Hospital, the chairman of the Cure Alzheimer’s Fund Research Consortium. “We were unlikely to get funding from the National Institutes of Health (NIH) to do this work at this early stage,” Stevens says. “It’s brand new research, still high-risk and high-reward. We need more pilot data to prove how promising it is.” At Tanzi’s recommendation, she submitted a proposal to Cure Alzheimer’s Fund, which awarded a grant to take the project to its next stage.
Now, Stevens’ lab is looking at tissue and cerebrospinal fluid samples from human patients with mild cognitive impairment (a diagnosis that often precedes Alzheimer’s) and early AD. They’re hoping to see evidence of the same abnormal levels of complement they witnessed in mice. If they do, they then will explore how to prevent the overproduction of complement in humans—and hopefully prevent cognitive decline.
What’s next
“Complement shows real potential as a therapeutic target,” Stevens explains. “It’s especially exciting because it’s involved at a very early point in the disease. If we could stop synapse loss early, we might be able to stop cognitive decline, or at least stave it off for several years.” Stevens is collaborating with fellow Cure Alzheimer’s-funded researcher Cynthia Lemere, Ph.D., to show that protecting synapses also protects against memory loss in mice. There are some challenges ahead, however. Since complement plays other important roles in the brain, a safe and effective therapy would need to regulate it without removing it completely. Stevens also anticipates hurdles with getting a therapy into the brain and making sure it acts specifically, targeting complement alone and no other proteins. “I don’t expect this work will overturn our basic disease model of amyloid, tau and inflammation,” Stevens says, “but it adds another layer.”
Tanzi is excited about the project. “This is a great example of what can happen when scientists from different disciplines collaborate with one another,” he says.
“This is groundbreaking work—exactly the kind of project Cure Alzheimer’s Fund is designed to support,” says Tim Armour, president and CEO of Cure Alzheimer’s Fund. “We’re helping Dr. Stevens to investigate a new idea that both enhances our understanding of Alzheimer’s and opens the door to new therapies. We’re eager to see where her work goes next.”