Posted March 15, 2019
There has been a great deal of news surrounding the research implicating gum disease in contributing to Alzheimer’s disease and dementia.
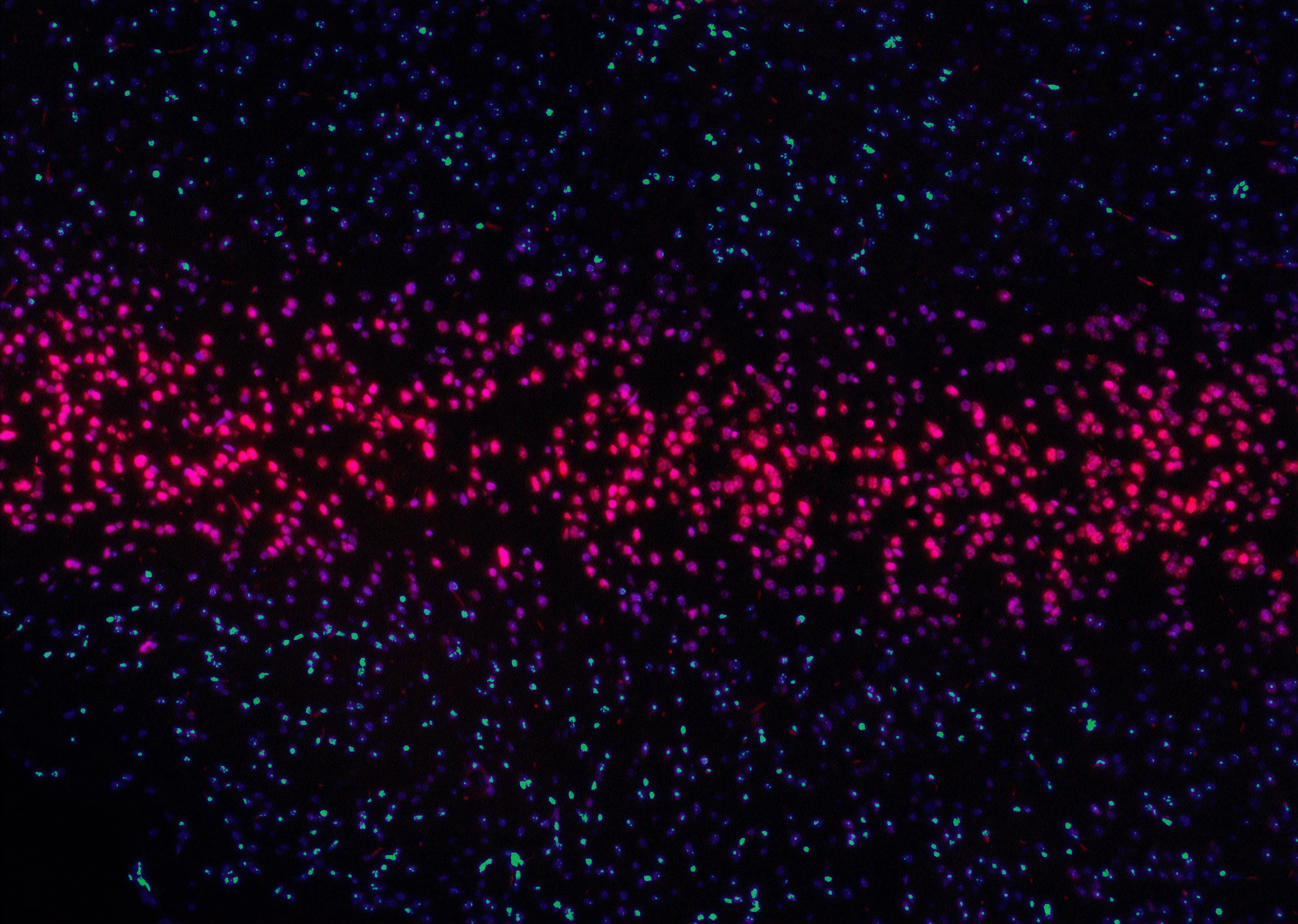
It is through the lens of the antimicrobial approach to treating Alzheimer’s disease that the latest study suggesting that periodontal bacteria can play a role in Alzheimer’s disease should be evaluated. The new research published on January 23, 2019 evaluated whether an oral infection with P. gingivalis can cause amyloid plaque build-up and neurodegeneration in mice and humans. The study examined the presence of bacterial DNA in post-mortem brain tissue from patients and the cerebrospinal fluid from people clinically diagnosed with Alzheimer’s disease.
This research provides more mechanistic insight into a specific microbe, P. gingivalis, which may be capable of initiating or aggravating Alzheimer’s disease pathology. However, it is premature to believe the claims by the media that regular visits to the dentist and good oral hygiene alone are the long sought after cure. While a deep dive into how the pathogen P. gingivalis antagonizes the brain is important, it should be in the context of cautious optimism given that the small-molecule inhibitor evaluated in the study has only completed phase I in clinical trials. In addition, other studies have not always found the presence of gum disease in individuals with Alzheimer’s and dementia.
A Deeper Dive Into The Research
In gum disease, P. ginvivalis is often resistant to antibiotics, which requires another player to be targeted: gingipains. Gingipains are toxic proteases (enzymes that breakdown other proteins) that are secreted by P. ginvivalis and are essential for the survival of microbe. Gingipains have been found in the brains of Alzheimer’s disease and the level of gingipain in the brain has been shown to correlate with the presence of toxic tau pathology. The hypothesis of the current study suggested that if you inhibit gingipain you could mediate the toxicity of P. gingivalis and prevent neurodegeneration.
The important research finding from this paper is that P. gingivalis and gingipains can spread from an infected oral cavity to the brain. The oral administration of small-molecule gingipain inhibitors has several important results:
The Antimicrobial Hypothesis and Alzheimer’s Disease
Drs. Rudy Tanzi and Rob Moir at Massachusetts General Hospital, through a grant provided by Cure Alzheimer’s Fund, have previously demonstrated that beta-amyloid has the potential to also play a protective role in the brain by trapping bacterial and viral invaders. As with most things in life, too much of a good thing can be bad. In the case of beta-amyloid, this protective build-up in response to infections in the brain – if left to its own devices without any inhibition – can then induce a build-up of amyloid plaques that is a hallmark of Alzheimer’s disease.
The Clinical Trails for Gingipain Inhibitors
The lead authors on the study are actively pursuing a small molecule inhibitor of gingipain. Gingipains make P. gingivalis more toxic to gums, and in its capacity as a protease also has been found to be neurotoxic. Gingipains have the capacity to kill neurons in cell culture and when injected into the memory center of the mouse brain, the hippocampus.
The Phase I clinical trial designed to examine safety and tolerance of CO4388 was completed in October of 2018. The Phase 1 study enrolled 33 subjects into 4 different cohorts. One of the cohorts enrolled 9 Alzheimer’s disease patients, and 6 of these patients received the small-molecule gingipain inhibitor called COR388. The primary conclusion of this study was that the drug was safe, and well tolerated. The Alzheimer’s patients in the study underwent exploratory cognitive testing to evaluate their performance on speech and language tests, memory, and reaction time.
Relevant Links:
Business Wire: Cortexyme Announces Phase I Data Demonstrating COR388 is Safe Well-Tolerated in Healthy Older Volunteers and Alzheimer’s Patients: https://www.businesswire.com/news/home/20181024005522/en/Cortexyme-Announces-Phase-1-Data-Demonstrating-COR388
Science News Magazine: “Gum Disease-Causing bacteria could spur Alzheimer’s.” https://www.sciencemag.org/news/2019/01/gum-disease-causing-bacteria-could-spur-alzheimer-s
Porphyromonas gingivalis in Alzheimer’s disease brains: evidence for disease causation and treatment with small-molecule inhibitors
http://advances.sciencemag.org/content/5/1/eaau3333/tab-pdf
For a more extensive review of the randomized, double-blind, placebo controlled, dose-escalation, first-in-human trial of the safety and tolerability of the small molecule gingipain inhibitor evaluated in the paper and referred to as COR388, please see the National Institute of Health website on the clinical trial: https://clinicaltrials.gov/ct2/show/NCT03331900?term=COR388&rank=2
Rudy Tanzi Tweet: https://twitter.com/RudyTanzi/status/1088628295531417601
Rob Moir Quote: https://www.sciencemag.org/news/2019/01/gum-disease-causing-bacteria-could-spur-alzheimer-s