Posted March 7, 2024

Engaging in physical exercise may bolster overall health and act as a safeguard for our brain, particularly with respect to Alzheimer’s disease (AD). Using a 3D cell culture model designed to mimic AD pathology, scientists discovered that the hormone irisin, which is released from the muscles during exercise, increases the production of neprilysin in specific brain cells known as astrocytes. Upon its release from astrocytes, neprilysin breaks down amyloid beta, the protein that forms harmful plaques in AD. Consequently, the levels of amyloid beta were significantly reduced. These results cast exercise and irisin in a new light as potential deterrents against the development of Alzheimer’s disease, and highlight a new therapeutic angle to prevent amyloid beta accumulation.
______________________________________________________________________________
Recent research has revealed a fascinating connection between physical exercise and Alzheimer’s disease (AD). It centers on a small protein called irisin. Produced during physical activity, irisin plays a pivotal role in brain health by potentially mitigating one of AD’s hallmark features—the accumulation of amyloid beta plaques.
Discovered in 2012, irisin gets its name from Iris, the Greek goddess who carried good news from the gods to humans. Echoing the role of its namesake, irisin also acts as a messenger, but in this case, as a chemical that ferries the beneficial effects of exercise throughout the body. Muscles release irisin into the bloodstream during physical exertion, and from there, irisin travels the body, carrying out its effects, such as converting white fat into energy-burning brown fat, strengthening bones and controlling sugar levels. Its reach even extends to the brain, where it is thought to sharpen cognitive abilities and play a role in AD prevention.
Irisin is present in both human and mouse brains, and its levels are lower in patients with AD and mouse models of the disease. Deleting the irisin gene in mice removes the cognitive benefits usually associated with exercise, partly because it interferes with the birth of new neurons in the hippocampus, a region of the brain important for learning and memory. On the other hand, in the same mouse models, increasing irisin levels in the bloodstream enhances cognition and reduces brain inflammation.
In addition to its ability to boost cognition in mouse models, physical exercise also reduces amyloid beta levels and inflammation in the brain. However, the connection between exercise, irisin and reduced amyloid beta levels is unclear.
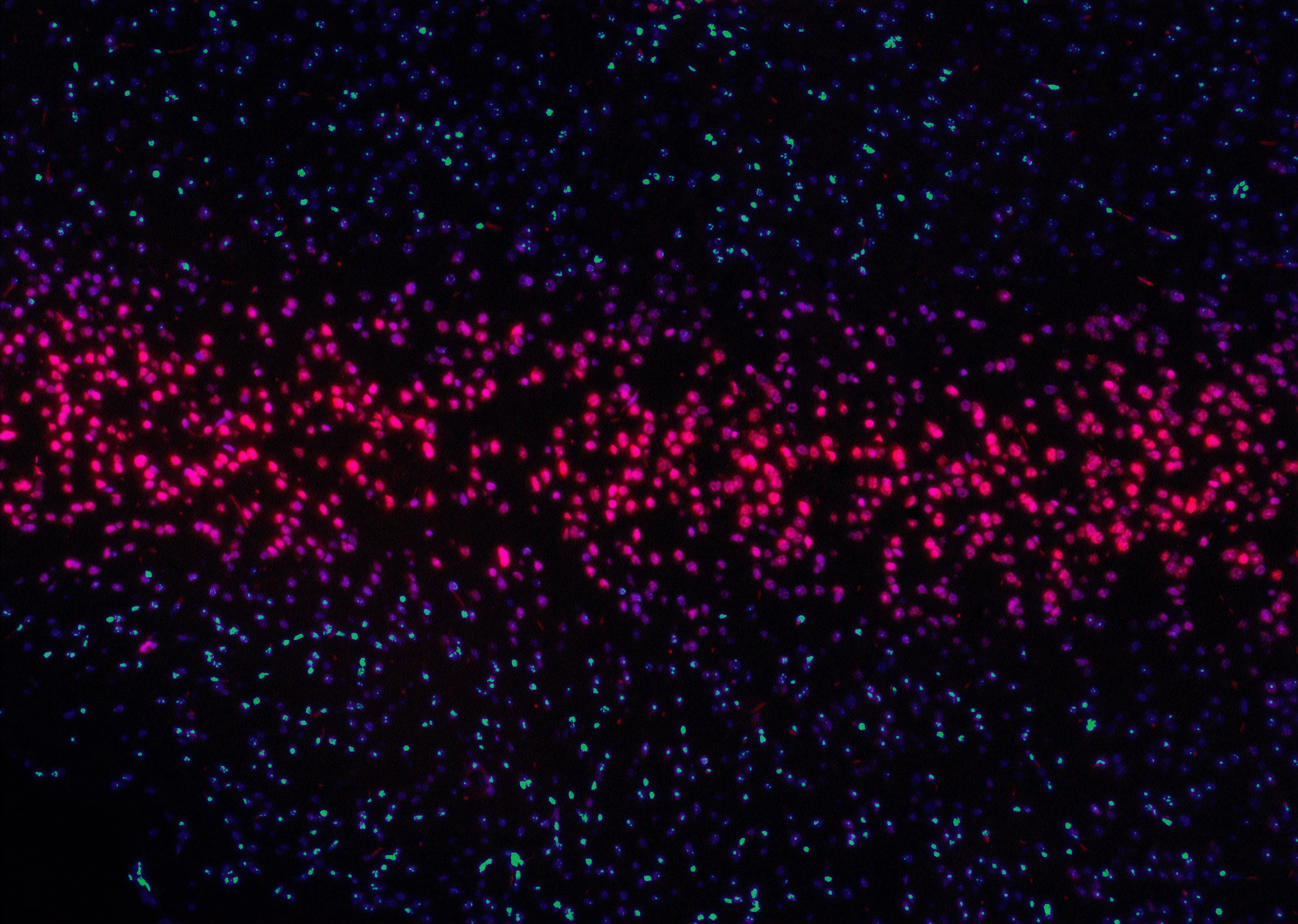
To determine whether there is a connection, scientists turned to a 3D cell culture model of AD known as Alzheimer’s in a DishÔ, created initially through a grant provided by Cure Alzheimer’s Fund. This model can be considered a mini-brain that fully replicates Alzheimer’s pathology, including amyloid plaques, tau tangles and neuroinflammation.
Introducing irisin to the model reduced amyloid beta levels by targeting brain cells known as astrocytes to produce more neprilysin, an enzyme that digests amyloid beta. Irisin binds to a specific receptor on astrocytes, which triggers a cascade of signaling events within the cell, culminating in the increased production of neprilysin. Astrocytes release neprilysin into their environment, allowing the enzyme to find and digest amyloid beta.
These findings suggest that increasing irisin levels in humans could combat the buildup of amyloid beta, which, according to the amyloid cascade theory of AD, eventually would prevent the trigger of tau tangles and cell death. The potential of irisin as a therapeutic agent is underscored by its ability to cross the blood-brain barrier. However, in the 3D cell models used in this study, irisin only decreased the amyloid burden in the earliest stages of the disease. When added to 3D cell models where the disease was more advanced, the levels of amyloid beta did not change. Therefore, treating patients with irisin in the early stages of the disease—or before symptoms appear—would be optimal.
This research reinforces the value of physical exercise in maintaining cognitive health and opens new pathways for developing treatments that leverage the body’s natural response to exercise. As scientists continue to unravel the complexities of AD and the potential of proteins like irisin, the hope is that such discoveries will lead to effective interventions for this debilitating disease.
Published in Neuron:
Joseph Park, Ph.D., Massachusetts General Hospital
Luisa Quinti, Ph.D., Massachusetts General Hospital
Doo Yeon Kim, Ph.D., Massachusetts General Hospital/Harvard Medical School
Christiane Wrann, D.V.M, Ph.D., Massachusetts General Hospital
Rudolph Tanzi, Ph.D., Massachusetts General Hospital/Harvard Medical School
Se Hoon Choi, Ph.D., Massachusetts General Hospital/Harvard Medical School