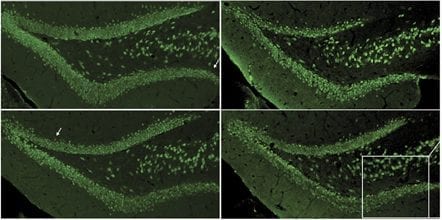
A promising first-in-class drug stimulates the creation of new nerve cells in the brains of Alzheimer’s mice and will soon be tested in the brains of human patients, thanks to new research by Dr. Sam Gandy, member of Cure Alzheimer’s Fund’s Research Consortium, at Mount Sinai School of Medicine in New York.
A new article by Gandy’s team just published in the journal Molecular Psychiatry outlines the extraordinary promise of the drug, known as a “mGluR2/3 blocker”. Created by the Japanese pharmaceutical firm Taisho and originally studied for depression, the drug acts by stimulating stem cells in the hippocampus to divide and form new nerve cells. What’s more, the learning behavior of the Alzheimer’s mice being treated with the mGluR2/3 blocker has been sustained at its normal level, in contrast to the steady decline of the mice not being treated.
mGluR2/3 originally caught the attention of Gandy and his team for its possible ability to inhibit production of the toxic protein Abeta42, which is associated with Alzheimer’s disease. With funding from Cure Alzheimer’s Fund, they conducted a pilot study of the drug’s effects on a particular strain of mice. That study turned out such promising results that it has drawn $1 million in funding from the Veterans Administration “MERIT Review” program that supports Gandy’s lab at the James J Peters VA Medical Center in the Bronx. Generous additional funding was also provided by the Louis B Mayer Foundation, the Sarah and Gideon Gartner Foundation, and the BrightFocus Foundation.
The mGluR2/3 blocker has also been administered to healthy young human subjects, and so far has been shown to be safe. The next step for Gandy’s team will be to treat elderly human subjects with the drug to test safety in this population before gearing up to test the drug in Alzheimer’s disease. With the focus of mainstream Alzheimer’s research turning toward prevention, the mGluR2/3 blocker is one of the few drugs that holds promise for repairing brains already damaged by neurodegenerative disease.
All of these efforts proceed from the international Stem Cell Consortium formed by Gandy in 2012 and funded by Cure Alzheimer’s Fund. “It’s extraordinary that in such a short time, we have moved from ordinary skin cells to induced pluripotent stem cells in a petri dish, to lab-generated human nerve cells, and now to a drug that could potentially create those cells inside a human brain,” said Gandy. “We realize that we are unlikely to have much impact in late-stage Alzheimer’s, but we are cautiously hopeful that this drug might arrest Alzheimer’s disease at an early stage so that patients can remain functional for more extended periods.” Gandy’s mGluR2/3 blocker is one of five brain cell regenerating agents currently undergoing testing in labs around the world.
“We are so proud of this development,” said Cure Alzheimer’s chairman Jeffrey Morby. “Helping incubate cutting edge science that can gain momentum with federal funding — this is precisely why Cure Alzheimer’s Fund exists.”
David Shenk is a senior advisor to Cure Alzheimer’s Fund and author of The Forgetting: Alzheimer’s: Portrait of an Epidemic.