Posted November 7, 2014

From the age of 2, when he disassembled his brother’s mechanical duck and his father praised him for his curiosity, Charlie Glabe was always interested in the way things work. In college he supported himself as a car mechanic, but found his true passion in scientific research. Today Charlie is a highly respected neuroscientist and a member of the Cure Alzheimer’s Fund Research Consortium.
Early days
Charlie Glabe was born in Columbus, Ohio, the second of three boys. When he was still a baby, his father moved the family to California for a position as a mathematics professor at Sacramento State College. His mother had been a high school English teacher, but after the move decided to stay home with her children.
In high school, Glabe’s science classes were “kind of bland,” he said. “Girls were much more interesting.” But when he attended Sacramento State College as a biology major, he discovered the joys of science. Glabe worked his way through college as a Volkswagen mechanic, which he now compares to research. “You’re under pressure to get the job done. You need to explore every possibility to figure out what’s wrong, eliminating the easiest things first. And it teaches you humility.” After getting his degree, Glabe wanted to work for the California Fish and Game Commission, but when he didn’t get the job (because he wasn’t a Vietnam war vet), he went to plan B: graduate school. “It turns out that I’m a better scientist than I ever would have been a bureaucrat.”
In 1973, Glabe attended the University of California’s (UC) graduate program to study cell biology, developmental biology and biochemistry. That’s when he met his wife, a family planning and domestic violence counselor, and built a life that came to include their three grown children. Glabe moved to the East Coast for a few years to become a postdoctoral fellow at The Johns Hopkins University School of Medicine, where he honed his skills as a biochemist. In 1980, he became a postdoctoral fellow at UC, San Francisco, then a staff scientist at the Worcester (Massachusetts) Foundation for Experimental Biology. In 1985, he returned to California as an assistant professor at UC, Irvine.
Alzheimer’s
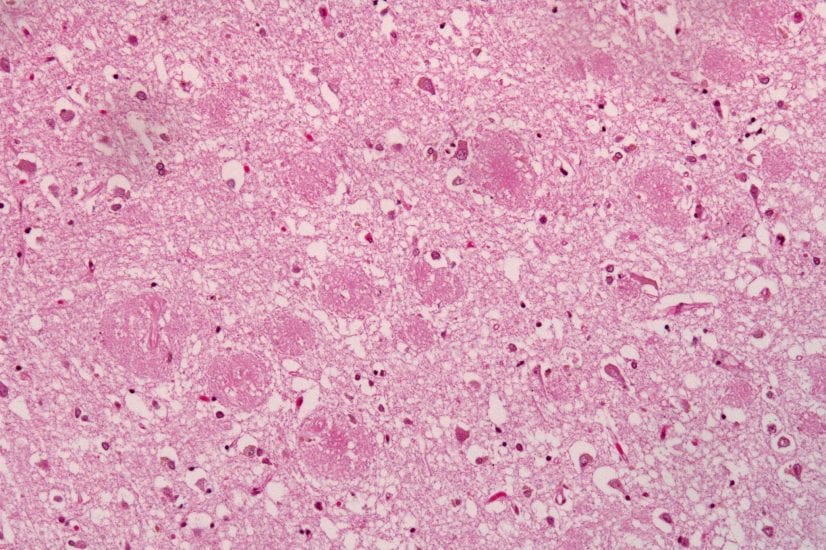
Glabe’s big break came later that year when he was working on the biology of marine animals. A friend of his who was working with the noted neuroscientist Carl Cotman asked Glabe for help with a very different experiment. They needed a particular peptide, or protein fragment, for their research. “He had the sequence written down on a piece of paper and said he’d give me $30,000 and a technician salary for a year to make it.” Glabe realized then this was no ordinary peptide; as it happened, he became one of the first people in the world to artificially manufacture Abeta, the famous toxic hallmark of Alzheimer’s disease. “My friend told me that Abeta caused Alzheimer’s and that I should be careful. So I started reading about it, and a light bulb went off in my head. This was something I wanted to work on,” said Glabe. “That’s when I became interested in intercellular amyloid. In 1992, we were looking for a receptor for a beta peptide. I thought it would be easy, but we soon discovered that the long form of Abeta was immortal, while the short form was easily degraded. In the process, we realized that cells could live for a long time despite Abeta accumulation—like a slow-growing tumor that ultimately takes over.”
In 2007, Glabe received a Cure Alzheimer’s Fund (CAF) grant to produce as many antibodies against Abeta as possible. (One promising strategy to combat Alzheimer’s is to use such antibodies as a vaccine to rid the body of the toxin as it accumulates.) Glabe originally had thought Abeta could fold up in only one way, but he soon discovered it could fold up in many different ways and that different antibodies can recognize and bind to these different foldomers. “This went against all our assumptions,” explained Glabe. “It turned out there were 23 antibodies—many more than we had expected—and we needed to test them right away before they died. I called [Cure Alzheimer’s Fund CEO] Tim Armour to ask for more money and he came through. It turned out that one of these antibodies reacted with a unique type of intranuclear amyloid that had never been seen before.” That finding charted Glabe’s path.
In 2010, the pharmaceutical giant Eli Lilly ran a clinical trial for Semagacestat, a gamma secretase inhibitor, as the first potentially disease-modifying drug for Alzheimer’s. Rather than helping the test group, the drug actually made them cognitively worse. Many companies working on similar drugs subsequently ended their Alzheimer’s programs, while many dedicated researchers—including Glabe—did a lot of soul searching. “When a drug has the opposite effect that you want it to have,” he said, “the simplest explanation is that we were thinking about the disease mechanism backwards.” He went back to the drawing board and developed a mirror image of the working amyloid hypothesis. “The Eli Lilly drug prevents the secretion of soluble Abeta, and we think that this causes the neuronal retention of insoluble Abeta,” Glabe explained. “These are mirror image mechanisms. When you decrease secreted soluble Abeta, you increase intraneuronal insoluble Abeta. That is the new amyloid hypothesis.”
His work ultimately supported the idea that gamma secretase modulators would be effective therapeutics vs. gamma secretase inhibitors, which only made patients worse. “You have to be fearless to say that 98 percent of people have been thinking about the disease backwards. But that’s the kind of research Cure Alzheimer’s Fund encourages,” said Glabe.
These days Charlie spends most of his time in his lab, although he teaches two classes a year. “I love science. I’m addicted to doing experiments and getting results. Everyone who’s part of the Cure Alzheimer’s Fund Research Consortium is doing cutting-edge work. And we have a diverse range of talent that proves that the whole is really greater than the sum of its parts.”