Posted March 14, 2017
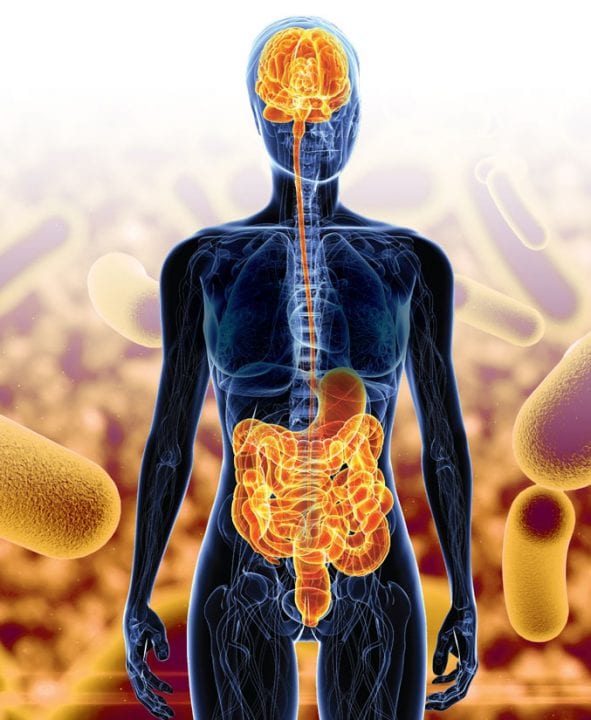
It may not be surprising to learn that brain health is intricately linked to the state of the rest of the body. But what are the links, and what role do these connections play in diseases like Alzheimer’s? Sam Sisodia, Ph.D., of the University of Chicago, is examining one of the most important connections: the way in which our gut microbiome influences the brain in Alzheimer’s disease.
The “gut microbiome” refers to the population of microorganisms, mostly bacteria, that lives in every person’s digestive tract. These are natural residents of our bodies, present even at birth. Each person’s microbiome is different, containing more of certain microorganism species and less of others. Sisodia is exploring the makeup of the gut microbiome and its broader impact on our health.
Sisodia is working with mice bred to exhibit the hallmark traits of Alzheimer’s disease: amyloid plaques, tau tangles, brain inflammation and loss of cognitive function. In a previous experiment, Sisodia treated some of these mice with antibiotics. As expected, these treated mice exhibited significant changes in their gut microbiomes. While overall bacteria levels remained the same, these mice had less bacterial diversity—certain species were no longer present, while others were present at elevated levels.
Sisodia was excited to see both fewer and smaller amyloid plaque depositions in the brains of treated mice. These mice showed higher levels of soluble amyloid beta and exhibited a different neuroinflammatory response from that of untreated mice. Inflammation in the brain is an immune response—an attempt by the body to fight an invading pathogen or to respond to other trauma—but it goes awry in Alzheimer’s disease as microglial cells become overactive, causing damage to nerve cells.
The antibiotic-treated mice showed lower levels of these cells around the remaining plaques. Sisodia suspects that the change in this inflammatory response might be the reason for the differences in Alzheimer’s pathology seen in the antibiotic-treated mice.
With these findings, Sisodia wants to know more. He now is testing the same antibiotics on a different strain of Alzheimer’s mice, making sure the effects he saw were not specific to the original mouse strain tested. He’s also investigating why changes in the gut microbiome might affect immune responses in the brain. “It’s fascinating to learn that what happens in our digestive tract can influence the pathology of Alzheimer’s in our brains, but if this work is going to lead us closer to a cure, we need to understand the mechanisms of action,” Sisodia said.
Sisodia’s work promises to uncover new insights into the causes and risk factors of Alzheimer’s disease, and hopefully identify targets for treatment. However, Sisodia strongly cautions against interpreting his work as a suggestion that antibiotics themselves might be a treatment for Alzheimer’s.
“I am investigating how gut microbial diversity impacts Alzheimer’s pathology, and antibiotics are a way to quickly alter that diversity in the lab—but my work does not support using antibiotics in human patients to address Alzheimer’s,” he said. “We need to learn more about the consequences of altering the gut microbiome, what an optimal microbiome population profile would be and how to achieve it. It’s possible some changes might actually accelerate amyloid deposition in the brain. What’s more, the overuse of antibiotics can have serious negative effects on the health of individuals and our population as a whole.”
Sisodia’s work also is deepening our understanding of one of the most critical components of Alzheimer’s: inflammation. “While plaques and tangles are better known, inflammation is what most directly causes nerve cell death,” he explained. “If we can figure out how to intervene in the processes that are responsible for the inflammation, we may be able to stop the disease from doing real damage to our brains.”
Dr. Sangram Sisodia was awarded a $250,000 grant by Cure Alzheimer’s Fund in 2016 for his project “Mechanisms By Which the Gut Microbiome Influences Amyloid Deposition and Neuroinflammation in Mouse Models of Alzheimer’s Disease.”